Prilosec OTC Product Monograph
Prilosec OTC Background
Prilosec OTC (omeprazole 20 mg as omeprazole magnesium 20.6 mg) has been approved by the U.S. Food and Drug Administration as an over-the-counter (OTC) medication for the treatment of frequent heartburn, defined as heartburn occurring two or more days a week. With Prilosec OTC, healthcare professionals have an OTC therapeutic option to recommend to adult patients who have frequent heartburn symptoms.
In the past, management options for self-treating consumers have included antacids and H2-receptor antagonists (H2RAs). Now consumers have access to PPIs including POTC, a long-lasting treatment option for controlling frequent heartburn symptoms—one dose a day works for up to 24 hours as part of a 14-day course of therapy. This section gives an overview about frequent heartburn sufferers and their treatment habits.
Characterization of Frequent Heartburn
Tight clothes can put pressure on your stomach, which can squeeze acid upward and into your esophagus.
Heartburn is described as a sensation of mid-chest discomfort moving up to the throat and neck, accompanied by a burning or painful feeling under the sternum. A 2003 survey showed that in the United States, about 65% of the total adult population experiences heartburn, with heartburn occurring daily in about 15 million adults.1a
Slightly more women (59%) than men report frequent heartburn.1a,2 The mean age for a consumer with frequent heartburn is 45 to 50 years,1a and heartburn has a slight tendency to increase with age.2 Geographic location, marital status, family status (children), education level, job type and level, and socioeconomic status all play a role in the tendency to develop heartburn.3
Figure 1. Frequency of heartburn in the U.S. heartburn population1a
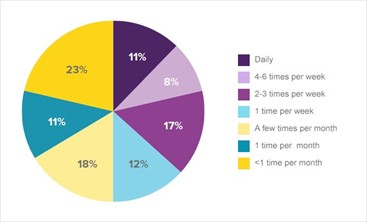
Consumers with frequent heartburn reported having a long history of heartburn symptoms. The majority of consumers with frequent heartburn have discussed their symptoms with healthcare professionals. Most consumers with frequent heartburn (62%) have reported their symptoms to their primary care physician; 16% and 2%, respectively, have seen gastroenterologists or cardiologists; and 30% have consulted with pharmacists.1a
OTC Heartburn Medications
Most consumers with frequent heartburn self-diagnose and self-treat using available OTC medications. In a 2001 survey, about 80% of individuals with frequent heartburn reported using OTC heartburn medications.1a
In this study, more than 70% of frequent heartburn sufferers considered their symptoms moderate to severe, and most frequent heartburn sufferers reported medicating at the first sign of symptoms to prevent them from getting worse or to relieve them. In general, consumers with frequent heartburn reported managing their heartburn by using antacids alone or in combination with OTC H2RAs, or prescription PPIs.1a
Prilosec OTC Description
Prilosec OTC is supplied in 14-tablet, 28-tablet, and 42-tablet sizes. These sizes contain one, two, and three 14-day courses of treatment, respectively. Prilosec OTC is a pink-colored (salmon) tablet consisting of multiple enteric-coated pellets formulated with 20.6 mg of omeprazole magnesium, equivalent to 20 mg of omeprazole. The active ingredient is the magnesium salt of omeprazole, which allows tableting. Prilosec OTC is also available in a Wildberry flavor tablet that provides a burst of flavor while swallowing the tablet. Prilosec OTC Wildberry is not meant to be chewed or sucked. It is a purple colored tablet and is available in 14 and 42 ct. sizes. Currently, this tablet formulation is marketed as an OTC product in Sweden and as a prescription product in more than 30 other countries.1a
Prilosec OTC Composition
Clinical Pharmacology
Clinical Efficacy Studies
Two well-controlled clinical studies involving 3,120 subjects support the use of a consecutive 14-day therapeutic regimen of omeprazole magnesium to treat frequent heartburn. Both studies were multicenter, double-blind, randomized, parallel, and placebo-controlled. Each study evaluated 10 mg and 20 mg doses of omeprazole magnesium for 14 consecutive days in subjects with heartburn two or more days a week.1a
The studies had a one-week placebo run-in phase to assess heartburn frequency. Eligible subjects were randomized into a two-week double-blind treatment phase to receive a single daily dose of either omeprazole magnesium 10 mg, omeprazole magnesium 20 mg, or placebo every day. Subjects took their daily dose of study medication each morning before breakfast.1a
The primary efficacy end point was "no heartburn over the previous 24 hours" (i.e., completely heartburn free for a full day). Efficacy was evaluated following the first dose of medication, on the last dose, and over 14 days of dosing during the double-blind phase.1a
A number of secondary efficacy end points were also studied following the first dose of medication and subsequent doses. These included "complete prevention of nocturnal heartburn" and "occurrence of no more than mild heartburn."1b
Primary Efficacy End Point Results
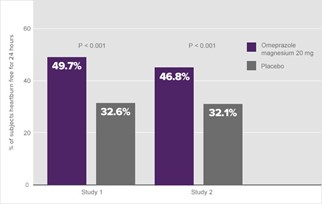
Both clinical studies showed that 20 mg of omeprazole magnesium resulted in a significant treatment effect during the first day. As shown in Figure 6, almost 50% of the subjects in the 20 mg omeprazole magnesium treatment groups were heartburn free for the full day after the first dose vs. approximately 32% of subjects in the placebo group (Figure 6).1a
Figure 6. Percentage of subjects with no heartburn for 24 hours—Day 11a
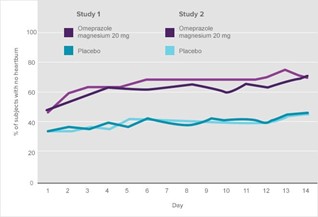
On Day 14, the percentage of subjects reporting complete heartburn relief was more than 70% (Figure 7).1a
Figure 7. Percentage of subjects with no heartburn for 24 hours—time course over 14 days1a
Secondary Efficacy End Point Results
In general, the results for these end points corroborated the findings for the primary end points.1a
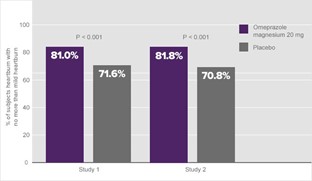
When subjects with only mild heartburn were added to the efficacy end point, more than 80% of those receiving omeprazole magnesium reported a significant therapeutic benefit on Day 1 (Figure 8).1a
Figure 8. Percentage of subjects with no more than mild heartburn—Day 11a
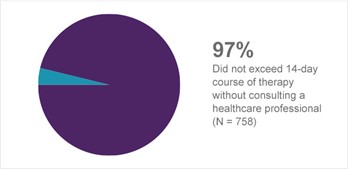
OTC Label Comprehension and Compliance
Compliance Study Results
Consumer behavior and understanding of the use of Prilosec OTC were evaluated in label-comprehension studies and an actual-use trial. This program of studies established the compliance with label directions and use of the product in an unsupervised setting. More specifically, the program was developed to determine whether consumers understood: 1) the population for which Prilosec OTC was best suited (self-selection based on frequency of heartburn and understanding of label warning language); 2) when and how to take Prilosec OTC (one tablet per day, 14 consecutive days); and 3) when to contact a healthcare professional (in response to specific warning language or when frequent heartburn returns). The actual-use study determined adherence to the label directions under conditions of actual use.1a
For each of the self-selection criteria, appropriate choice was greater than 90% across the population.1a
Subjects who elected to use the product were highly adherent to label dosing instructions, with more than 91% of subjects using the product as specified on the label by taking no more than one tablet per dose and no more than one tablet per day.1a
At a three-month follow-up interview, consumers who experienced a return of heartburn symptoms continued to display behavior consistent with label-use directions.1a
The results of these studies strongly support the appropriate use of Prilosec OTC by the consumer with frequent heartburn and support the ability of the consumer to correctly use the product within the proposed OTC label directions (Figure 9).1a
Figure 9. Dosing compliance1a
Indication and Usage
Prilosec OTC 20 mg tablets are indicated for the treatment of frequent heartburn, which is defined as heartburn occurring two or more days a week. The label for Prilosec OTC gives the following directions for appropriate use by consumers with frequent heartburn5
Safety
Contraindications
Prilosec OTC is contraindicated for those who are hypersensitive to omeprazole. Consumers who have certain medical conditions and/or symptoms are instructed by the label to either not use the product or to consult a doctor.5
Drug Interactions
The potential metabolic drug interactions with omeprazole (metabolized primarily by hepatic cytochrome P450 isoenzyme CYP2C19) have been systematically studied, especially with respect to warfarin, diazepam, digoxin, and clopidogrel.4
FDA Health Advisory Issued
Learn why data recommends avoiding the combination of Prilosec OTC (omeprazole) and Plavix® (clopidogrel).
While clinically significant interactions between warfarin or digoxin and omeprazole are unlikely, the narrower therapeutic window for these drugs led to the conservative precaution of listing them on the label for Prilosec OTC.4
In addition, FDA has required labeling warning patients to not use POTC if they are taking: cilostizol, prescription antifungal or anti-yeast medicines, diazepam, digoxin, tacroimus, mycophenolate mofetil, prescription antoretrovirals and methotrexate.
Warfarin Post-marketing reports of changes in prothrombin measures have been received among patients on concomitant warfarin and omeprazole therapy. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with PPIs and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time.
Diazepam Coadministration with diazepam was also listed in the Warnings section because omeprazole significantly reduces diazepam clearance, although the relatively wide therapeutic window for diazepam makes it unlikely that this effect of omeprazole is clinically significant.4
Antiretroviral Agents Concomitant administration of omeprazole has been reported to affect the plasma levels of antiretroviral agents, thus appropriate clinical monitoring is recommended.
Methotrexate Data appears to indicate that the drug-drug interaction between omeprazole and methotrexate may be linked to decreased elimination of methotrexate, leading to methotrexate toxicity. The approved labeling for methotrexate prescription products and all PPI prescription labels include this information.
Tacrolimus Concomitant administration of omeprazole and tacrolimus may increase the serum levels of tacrolimus.
Antiretroviral Agents Concomitant administration of omeprazole has been reported to affect the plasma levels of antiretroviral agents, thus appropriate clinical monitoring is recommended.
Methotrexate Data appears to indicate that the drug-drug interaction between omeprazole and methotrexate may be linked to decreased elimination of methotrexate, leading to methotrexate toxicity. The approved labeling for methotrexate prescription products and all PPI prescription labels include this information.
Tacrolimus Concomitant administration of omeprazole and tacrolimus may increase the serum levels of tacrolimus.
Drugs with pH-Dependent Absorption As with other PPIs and H2RAs, omeprazole increases intragastric pH (decreases acidity), which can affect the absorption of drugs that have pH-dependent absorption, e.g., ketoconazole, itraconazole or mycophenolate mofetil. A study demonstrated that ketoconazole absorption was greatly decreased after administration of omeprazole. Similarly, another study showed that itraconazole absorption was decreased if administered after a two-week regimen of omeprazole.4
Co-administration of mycophenolate mofetil with a PPI results in reduced systemic exposure of mycophenolate mofetil based on studies cited in literature.11 This effect is possibly due to gastric acid suppression by the PPI which may lead to incomplete dissolution of mycophenolate mofetil and subsequent poor absorption. This information is included in the current mycophenolate mofetil prescription labels.
Therefore, the label for Prilosec OTC lists potential drug interactions with these drugs under the Warnings section of the drug facts box.5
Special Populations Use in Pregnancy and Lactation: The label for Prilosec OTC directs pregnant or lactating women to consult a physician before use.5
Use in Children: Prilosec OTC is indicated for the treatment of frequent heartburn for those aged 18 years or older. For children younger than 18 years with frequent heartburn, the label instructs consulting a physician before using.5
Adverse Events Doctors have prescribed omeprazole to millions of patients to treat acid-related conditions safely.7
The safety of omeprazole was confirmed in 15 new OTC clinical trials with omeprazole magnesium (n > 18,000). The most common adverse events were headache and diarrhea, consistent with those in clinical trials and post-marketing surveillance for prescription Prilosec. Prilosec OTC had a similar tolerability profile to placebo.1a
Availability and Storage
Prilosec OTC tablets in a 20 mg dose are provided in blister packs.
Prilosec OTC is available OTC in three different sizes5:
- Package of 14 tablets (one 14-day treatment course)
- Package of 28 tablets (two 14-day treatment courses)
- Package of 42 tablets (three 14-day treatment courses)
Prilosec OTC Wildberry Flavor tablets a 20 mg dose are provided in blister packs.
Prilosec OTC Wildberry Flavor is available OTC in two different sizes5:
- Package of 14 tablets (one 14-day treatment course)
- Package of 42 tablets (three 14-day treatment courses)
Storage Conditions
- Store at 20°C to 25°C (68°F to 77°F).5
- Protect from moisture.5
References
1a Data on file. Procter & Gamble.
1b Comparison of Prilosec OTC™ (omeprazole magnesium 20.6 mg) to placebo for 14 days in the treatment of frequent heartburn. Journal of Clinical Pharmacy and Therapeutics. 2005;30:105-112.
2 Oliveria SA, Christos PJ, Talley NJ, et al. Heartburn risk factors, knowledge, and prevention strategies: a population-based survey of individuals with heartburn. Arch Intern Med. 1999;159:1592-1598.
3 Profile of consumers in need. When the South rises again, it's probably just gas: a light-hearted look at heavy stomachs. Progressive Grocer 1995;98-99. 4 Data on file. AstraZeneca LP.
5 Prilosec OTC [package label]. Cincinnati, Ohio: Procter & Gamble; 2015.
6 Massoomi F, Savage J, Destache CJ. Omeprazole: a comprehensive review. Pharmacotherapy 1993;13:46-59.
7 Prilosec [package insert]. Wilmington, Del: AstraZeneca LP; 2015.
8 Lindberg P, Brändström A, Wallmark B, et al. Omeprazole: the first proton pump inhibitor. Med Res Rev. 1990;10:1-54.
9 Lind T, Cederberg C, Axelson M, et al. Long-term acid inhibitory effect of different daily doses of omeprazole 24 hours after dosing. Scand J Gastroenterol. 1986;21(suppl 118):137-138.
10 Losec [package insert]. Auckland, New Zealand: AstraZeneca Limited; 2015.
11 The role of proton pump inhibitors on early mycophenolic acid exposure in kidney transplantation: evidence from the CLEAR study. Kiberd BA, Wrobel M, Danavino R, et al. Ther Drug Monit. 2011 Feb; 33(1):120-3; Proton pump inhibitors reduce mycophenolate exposure in heart transplant recipients – a prospective case controlled study. Kofler S, Shvets N, Bigdeli AK, et al. Amer J. Transplant. 2009 Jul; 9(7):1650-6. Epub 2009 June 10.
With one Prilosec OTC pill in the morning, block your heartburn all day and all night.

†Pharmacy Times 2025-2026 Survey of Pharmacists’ OTC Recommendations
* Zero heartburn is possible with Prilosec OTC. Not for immediate relief. Take one pill per day as directed to treat frequent heartburn. May take 1 to 4 days for full effect. Do not take for more than 14 days or more often than every 4 months unless directed by a doctor. Use as directed.
** PG Calculation based in part on Buying Households reported by the Nielsen Company through its Homescan Panel service in the US for Prilosec OTC for the period of 8/31/03 through 6/25/16